Fibromyalgia Basics
Savella for Fibromyalgia
Input from Doctors & Patients
![]()
Savella (milnacipran) was approved for treating fibromyalgia by the Food and Drug Administration (FDA) in 2009. Unfortunately, it doesn’t go off patent until 2029. Out of all your medication options, this drug is likely the most expensive. Based on cost alone, you ought to ask: Is it worth the money to give it a try? In addition, you should also ask: Is there a strategy for determining who might respond to Savella?
Like the other two FDA-approved drugs for fibromyalgia, Savella reduces pain in a minority of patients. But on the upside, the drug can potentially reduce fatigue. According to a 2021 report, the patients most likely to benefit from Savella are those without anxiety or problems sleeping.1 However, the absence of these two symptoms does not guarantee a favorable response to the drug.
The FDA trials enrolled people with mild cases of fibromyalgia who did not have any other painful medical condition. Clearly, this is not the average patient, so the results may not apply to you. That’s why a survey of 503 “typical” patients who tried Savella for fibromyalgia offers useful insight on the drug’s tolerability. In addition, several doctors with experience using the medication offer advice on navigating Savella’s side effects.
How it Works
Savella works in the central nervous system to increase norepinephrine (NE) and serotonin at the nerve junction. It’s in the same class as duloxetine, a selective serotonin and NE reuptake inhibitor or SNRI. Basically, these drugs allow NE and serotonin to remain available at the nerve junction to be reused. Savella exerts three times the amount of action on NE compared to serotonin. By comparison, duloxetine affects these two transmitters equally. The increased action on NE is why the drug can relieve fatigue and prevent weight gain. However, the stronger impact on NE is tied to several undesirable side effects.
When impulse signals from painful muscles enter the spinal cord, various centers in the brain release NE, serotonin, and opioids to tone down or filter out the pain inputs. These brain centers are underperforming in fibromyalgia. This is why medications that increase the action of NE and serotonin are prescribed.
One study measured the effectiveness of the pain inhibitory system in patients before and after taking Savella for one month, compared to patients taking a placebo.2 Twelve percent of the fibromyalgia patients on 100 mg/day of Savella showed a measurable improvement in the function of their pain inhibitory functions. Perhaps if the trial was longer, more patients would have responded favorably to the drug. Still, this doesn’t explain why the drug fails to benefit most patients.
What You Should Know
The clinical trials supporting the FDA’s approval of Savella for fibromyalgia tested two daily doses: 100 mg and 200 mg. Due to the drug’s quick elimination from the body (half of it is gone in 6-8 hours), Savella must be taken in two equally divided portions a day.
In the trials, roughly 20 percent of the patients taking Savella (either dose) were deemed responders, compared to 12 percent in the placebo group.3 This is a small margin of difference. Fatigue, cognition (fibrofog), and mood also improved for those taking the drug.
Slowly titrating from the lowest dose of 12.5 mg allows the body to build up tolerance to the drug’s side effects. Robert Ettlinger, M.D., of Tacoma, WA, says, “I find the ‛start low, go slow’ approach to dose increases is a good strategy. By titrating slowly, I discovered that many patients do well on 25 mg twice a day. Why push the dose up over days or weeks and possibly get into side-effect trouble? Most patients have had the disease for years and can gradually adjust to the medication over months.”
What are the most common side effects of Savella? “The nausea is the biggest problem with this drug,” says Daniel Clauw, M.D., of the University of Michigan at Ann Arbor. Commenting further, he adds: “This is a class effect, as duloxetine has the same issues. The nausea is better if people take it with plenty of food, and it often resolves within a few weeks.”
Savella for Fibromyalgia
What Are the Odds it Helps?
One-fourth of the 503 patients surveyed who tried Savella rated the drug as beneficial for treating pain and fatigue. Eighty percent of the survey participants were at a dose of 100 mg/day or less.
The greatest factor predicting a person’s response to Savella had to do with the severity of the drug’s side effects. Patients experiencing mild side effects were four times more likely to benefit from Savella than those who quit the drug due to intolerable side effects.
Many of the patients surveyed said the titration recommendations to increase to the target dose were too aggressive. In hindsight, this likely leads to more side effects. So, for anyone who wishes to try Savella, the advice that follows ought to make it a smoother experience.
Minimizing the Side Effects
“In my experience, about 75 percent of patients tolerate the drug with little or no nausea,” says Kevin Hackshaw, M.D., now at University of Texas in Austin. “When patients encounter this side effect, it can be severe (surpassing that of duloxetine).”
“If tolerated, a three-week titration period is required as a minimum. It may need to be expanded to six weeks to build tolerance to the side effects. Major symptom improvements can be seen as early as one week in most cases. In a smaller subgroup (10-25 percent), patients need to go through the entire six-week titration period. In addition, they might require dosing up further to 100 mg twice daily to reap the desired efficacy.”
Many of Savella’s side effects can be manageable by altering the way you take this drug, but not always.
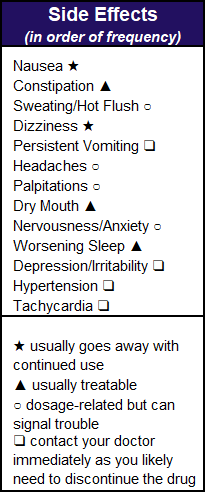
Nausea – Savella is best taken on a full stomach (not just milk and cookies) and the nausea may persist. “Some patients find they get benefit from the drug at 25 mg twice daily with less nausea,” says Hackshaw. “Although these patients may get more efficacy at 50 mg or 100 mg twice daily, they are unable to tolerate the degree of nausea.”
Kenneth Miller, M.D., a clinical trial expert in Danbury, CT, has patients with similar experiences. “Patients often reach the 25 mg twice daily dose in a few days but encounter more GI symptoms. They should stay at this dose until the GI symptoms improve. Then they should increase the evening dose to 50 mg for one week and move up to 50 mg twice daily.”
Another approach for taking Savella for fibromyalgia is offered by Kim Jones, Ph.D., FNP, now at Emory University in Atlanta. “Start by taking a 12.5 mg tablet with the evening meal, only once daily.” There is no reason why you can’t titrate up in smaller increments to improve tolerability.
Dizziness – “This symptom tends to get better over three months of continued use,” says Ettlinger. To get through this acclimation period, Hackshaw suggests omitting the morning dose and only taking Savella in the evening for a week before trying it twice daily again. You can even get creative with dosing (off label). For example, take the bulk of the drug in the evening because this symptom isn’t an issue while sleeping.
Worsening of Sleep – Jones suggests that you cut your evening dose in half (off label). “If this doesn’t work, add pregabalin at bedtime. If you still aren’t sleeping, add a sleep medication.” A strategy endorsed by the fibromyalgia patients surveyed (most of whom were already on a sleep medication) is to take the evening dose at least six hours before bedtime.
Possible Deal Breakers
Sweating and Hot Flush – “These symptoms are not unique to Savella; they occur with duloxetine as well,” says Luis Torregrosa, M.D., in Dearborn, MI. “There are no effective therapies for the sweating side effect.” Lawrence Robbins, M.D., a neurologist in Northbrook, IL, agrees with Torregrosa and adds, “Sweating is one of the side effects that may not reduce with continued use.” If the sweating is too much, Savella is not a good medication for you.
Palpitations (pounding heart) – “This symptom can occur with any medication that increases NE,” says Robbins. “It depends upon how annoying or severe the palpitations are. In general, though, it may signal the need to discontinue the drug.” Hackshaw agrees, especially if the symptom persists at the 12.5 mg dosing. “Palpitations are usually dosage-related and don’t go away unless the dosage is decreased, or the drug is stopped.”
Headaches – “This symptom appears to be dosage-related, and reducing the dose in the morning often minimizes headaches,” says Hackshaw. He also suggests that omitting the morning dose for a week sometimes helps. “If headaches increase,” says Robbins, “Savella usually must be discontinued. It is not a good idea to treat the side effects of one drug with another drug unless we can’t help it.”
Nervousness/Anxiety – “The anxiety initially experienced on Savella often lessens with continued use,” says Robbins. However, if you already have moderate anxiety or serious insomnia problems, you might need to ditch this drug. On the other hand, Ettlinger suggests lowering the dose for a period to see if tolerance develops.
Signs of Trouble
Savella should probably be discontinued when the following symptoms persist at the lowest dose beyond two weeks: nausea, headaches, palpitations, and sweating. If the nausea leads to regular vomiting, Miller recommends discontinuing the drug. He also says that tachycardia (rapid heartbeat) and hypertension (significantly elevated blood pressure) are also signs that this medication is not for you.
Depression/Irritability – “Although Savella is not FDA-approved for treating depression, it’s an antidepressant,” says Lesley Arnold, M.D., of Cincinnati Ohio. “It carries a black box warning about suicidal thoughts that is the same for other antidepressants. A worsening of mood or suicidal thoughts while taking Savella should prompt patients to contact their doctor immediately and they will probably have to discontinue the drug.” Arnold also brings up an important but delicate subject: bipolar mood disorder.
If your mood worsens with an antidepressant, including symptoms of agitation or irritability, Arnold says you may have bipolar disorder. She recommends you be evaluated for this condition because medications for this illness are different. Patients should be aware of the possibilities, says Arnold. That way, “alternatives to antidepressants can be considered for both pain and mood treatment.”
Robbins suggests that some people with bipolar might tolerate Savella if they are also on a mood stabilizer such as lamotrigine (Lamictal).
Drug Interactions
Most medications you take are broken down by enzymes in your liver so that your body can eliminate them. Only a small portion of Savella is processed through the liver, and it has no effect on your liver enzymes.4 So even if you are taking multiple medications, you don’t have to worry about Savella causing serious drug interaction. You do, however, need to limit your exposure to medications that increase serotonin. Too much of this transmitter leads to a toxic condition called serotonin syndrome.
Before you begin taking Savella, you will probably have to discontinue or lower the dose medications that raise serotonin. Examples include selective serotonin reuptake inhibitors (SSRIs), certain tricyclic antidepressants, and tramadol. If you are on duloxetine, Arnold says it is possible to taper the dose of duloxetine while slowly increasing Savella. Robbins says the triptan class of drugs used to treat migraines could produce a serious reaction. The point is, you only need to discontinue (or taper) serotonin enhancing drugs, but you can stay on other medications that do not alter this chemical.
For more information about other medications for fibromyalgia, see medications.
If you are interested in alternative therapies, see nondrug treatments.
To read about the role of diet for easing fibromyalgia symptoms, see Diet & Nutrition.
1. Gupta H, et al. Health Psychol Res 9(1), 2021. Free Report
2. Pickering G, et al. Drug Des Dev Ther 12:2485-2496, 2018. Free Report
3. Mease PJ, et al. J Rheumatol 36:398-409, 2009. Abstract
4. Puozzo C, et al. Clin Pharmacokinet 44:977-88, 2005. Abstract
