Fibromyalgia Basics
Transcranial Magnetic Stimulation
–TMS for Fibromyalgia
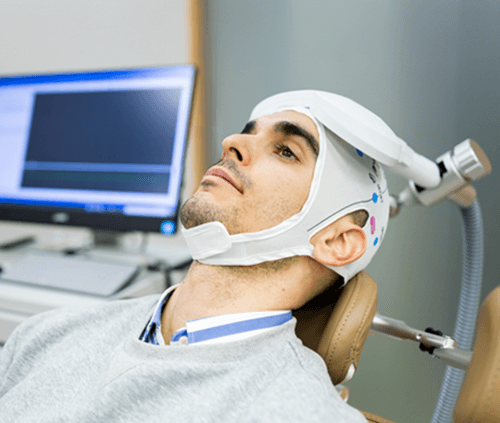
TMS is the most effective yet non-invasive way to stimulate brain activity in a targeted region with minimal side effects. It’s FDA-approved to treat depression, OCD, migraines, and to help people discontinue smoking. Studies show TMS also treats other medical conditions, such as Parkinson’s disease, Alzheimer’s disease, neuropathic pain, and yes, fibromyalgia. Unfortunately, insurance companies won’t pay for these conditions, at least not yet.
How TMS Works
Patients sit in a recliner while a figure-eight or cone-shaped device is placed on their head. A coil inside the device transmits a magnetic field through the skull into a targeted brain region. The goal is to favorably alter the brain’s cortical activity in a specific area as well as the connecting circuits. The transmissions are repetitive pulses and the TMS computer controls the speed (frequency), intensity (power), and duration of the session. These variables depend on the condition being treated.
TMS treatments change neuron function, enhance cerebral blood flow, and facilitate cellular regeneration.1 TMS also reduces the activation of the brain’s immune cells (astrocytes and microglia) to suppress neuroinflammation. Well, at least this latter effect occurs in rodent models of several diseases. In a way, the therapy restores the brain’s normal functions that are disrupted by the presence of a disease.
Standard TMS therapy is performed five days a week for four to six weeks. However, recent technological improvements are paving the way to shorter protocols requiring only 3-5 consecutive days with multiple treatments per day. Also, studies show that the favorable brain alterations last between six months to two years. As for side effects, they are short-lived and minimal.
The Upside
Hundreds of neuroimaging studies in fibromyalgia patients reveal a malfunctioning brain. If you take a medication to change the way your brain works, this exposes your body to the drug’s systemic side effects. On the other hand, TMS is a more precise method to get your brain’s neural circuits to operate correctly.

Putting aside the favorable impact on the brain’s neural networks, TMS improves cerebral blood flow which is impaired in fibromyalgia. And theoretically, it also tames the brain’s immune cells, which are in an activated state and causing neuroinflammation.
Your brain is not the only system disrupted by fibromyalgia, but it plays a key role in generating your symptoms. Many peripheral factors, such as your immune cells and gut bacteria, drive your brain’s malfunctions. Correcting the brain’s abnormal control processes prevents these peripheral drivers from making matters worse.
The Downside
TMS sounds like the ideal therapy for fibromyalgia patients. So, why hasn’t your doctor prescribed it for you? It’s not FDA-approved for treating fibromyalgia, let alone any chronic pain condition. Without the FDA’s endorsement, insurance companies refuse to pay and the cost runs around $10,000. But scientists are working to show TMS is a viable treatment option worthy of FDA approval for fibromyalgia.
Fibro Studies
The potential of TMS to treat pain caught the eye of fibromyalgia researchers 15 years ago. Now, more than a dozen clinical trials involving fibromyalgia patients (both men and women) are published.2 Most of the trials target the motor cortex, an area at the top of your brain (in line with your ears). But why would changing the neuron communications in the motor cortex help reduce your pain?
Just as the name implies, the motor cortex controls movement, but it also has extensive connections to other brain networks. Some of these networks play a role in regulating pain, stiffness, and sleep. Indeed, treating the motor cortex to relieve neuropathic pain prompted researchers to test this area for fibromyalgia.
The largest TMS trial involving 100 fibromyalgia patients (all female) shows tremendous promise.3 Half the participants were administered the “active” treatment. The other half were given a “sham” treatment. Patients in this group were hooked up to the machine but without power to the magnetic coil. This design ensures that any favorable results cannot be chalked up to a placebo response (i.e., patients’ eagerness to feel better).
The trial involved daily treatment for the first ten days, followed by six weekly sessions. Each day of treatment included one 15-minute therapy session (i.e., not multiple sessions per day). Patients were evaluated before the study, after the last treatment session, and two months later.
At the end of the weekly sessions, pain levels dropped in half for 40 percent of the patients in the active group, compared to 18 percent in the sham group. The functional improvements remained at the two-month follow-up although a portion of the pain returned. In addition, many patients in the active group reduced their drug use, but not those in the sham group.
Fine-Tuning Therapy
The sham-controlled trial shows that TMS provides real benefits for women with fibromyalgia—beyond a placebo effect. Future studies should include men to broaden the findings. Keep in mind, researchers launched this study seven years ago, and the technology has advanced significantly since then. Additionally, clinicians can fine-tune the protocol in several ways to achieve even better results.
The motor cortex is the target area for treating neuropathic pain, but another region might work better for fibromyalgia. Just changing the treatment area can dramatically improve the efficacy.
The motor cortex, or any brain center, is relatively large and no two brains are alike. So, if you target an area, it is helpful to use functional MRI on each person to identify the region with the least amount of activity. Then the TMS coil is applied to stimulate that specific region. It’s a technique called brain-navigation and enables more precision therapy.
Advances in technology allow for increases in daily doses of TMS therapy. Instead of receiving just one treatment session, patients receive multiple treatments each day. Research on depression shows that this accelerated protocol delivers faster results and longer-lasting benefits.4
Targeting a more fibromyalgia-appropriate area using brain-navigation and the accelerated protocol will improve efficacy. But customizing the TMS technology to treat fibromyalgia is a process. Fortunately, AFSA funded a study at Duke University to expedite progress. In addition to perfecting the protocol, the study examines how this technology exerts its symptom-reducing effects. For more details, see TMS Trial in Fibromyalgia.
Don’t Miss a Beat on New Treatments & Research: Sign up for a Free Membership today.
What Causes Fibro | Brain Inflammation | Diet & Nutrition | Muscle Pain Relief | Medications
References for TMS & Fibromyalgia
- Szymoniuk M, et al, Neurosurgical Rev 46(1):127, 2023. Free Report
- Velickovic Z, Radunovic G. J Pers Med 14(6):662, 2024. Free Report
- Silva VA, et al. BJA 134(6):1756-1764, 2025. Free Report
- Roth Y, et al. Psychiatry Res 328:115482, 2023. Free Report
