Possible Causes
Hyped-Up Ganglia
…Source of Fibromyalgia Pain
Before signals enter your spinal cord, they pass through the dorsal root ganglia (DRG), structures believed to amplify fibromyalgia pain. The DRG are tiny bulb-shaped units containing the cell body of each neuron and they are surrounded by a glial cell (called a satellite glial cell or SGC). The DRG/SGC complex samples information from the blood and lymph and uses it to influence the central nervous system (CNS).1,2
![]()
If the DRG/SGC receives distress signals from the periphery, such as an injury or an infection, it conveys that information to the cord. The DRG/SGC complex tones down signals with GABA or amplifies them with substance P (and other pain transmitters). This keeps your brain in the loop about what is happening in your body.
Although the ganglia reside in your periphery, the fluid space between the DRG and the glial cell is shared with the cerebral spinal fluid in the cord. So, the DRG/SGC is uniquely positioned to balance the information from your periphery with the CNS.
Ganglia Studies in Fibromyalgia
For decades, fibromyalgia researchers ignored the DRG/SGC complex because it cannot be analyzed by modern neuroimaging techniques. Well, at least not in humans. However, scientists today are more creative.
Injecting mice with various cells from fibromyalgia patients highlights the ganglia’s important role in this disease. Indeed, passively transferring fibromyalgia cells to mice causes a dramatic increase in pain sensitivity. And evaluation of the mice reveals the DRG/SGC structures are involved.
Unlike humans, the DRG/SGC in mice can be imaged. In addition, the strength of the signal generated by this unit can be measured before and after giving the mouse a pain stimulus. As a result, transfer studies provide insights into how the DRG/SGC can impact CNS function and cause fibromyalgia symptoms.
A closer look at the ganglia and the cells that impact them will improve our understanding of fibromyalgia. In turn, this will generate novel treatments for fibromyalgia, as opposed to those that solely target the CNS.
So far, three passive transfer studies involving fibromyalgia patients emphasize the role of the ganglia. Substances in the periphery appear to make the DRGs behave differently, such that the signals entering the spinal cord are chronically amplified. This could make the CNS falsely think that it is fighting a widespread infection or something equally serious.
Passive Transfer Studies
Immunoglobulins Surrounding the DRG: Could antibodies in the serum of fibromyalgia patients be attacking the glial cells that envelop the DRGs? If this is the case, it implies that fibromyalgia is a very different type of autoimmune disease. Instead of targeting the joints, muscles or organs, the DRG/SGC complex might be the focus of the immune system’s involvement in fibromyalgia.3 Read more.
Misbehaving Neutrophils: Transfer of neutrophils from fibromyalgia patients to mice leads to a rapid increase in the animal’s pain sensitivity. Imaging the mice shows the neutrophils invading the DRG, but this doesn’t happen with neutrophils from healthy people.4 Apparently, the neutrophils from fibromyalgia patients have undergone changes that can activate the ganglia and ramp up pain. This is a promising new area of research. In fact, AFSA funded a study in July 2025 to explore the role of neutrophils in fibromyalgia patients (not mice). Read more.
Altered Gut Microbes: The microbes living in your gut (called your microbiota) do more than help digest your food. They also communicate with your CNS by activating substances in your immune system. Unfortunately, the microbiota of fibromyalgia patients is different from that of healthy pain-free people. In fact, the greater the imbalance of microbes, the greater the pain and other symptoms. Implanting gut microbiota from fibromyalgia patients into the gut of mice makes them pain sensitive, partly due to its effect on the ganglia. However, the microbiota transfer also alters the mouse’s metabolic and immunologic processes as well as many different cells in the CNS.5 Read more.
Implications for Fibro
Andreas Goebel, M.D., Ph.D., in collaboration with Eva Kosek, M.D., Ph.D., published the first fibromyalgia passive transfer study in 2021. Two other reports looking at neutrophils (Shafaq Sikandar, Ph.D.) and gut microbes (Amir Minerbi, M.D., Ph.D.) followed. They are proof that something in the blood or peripheral systems drives your pain and the dysfunctions in your CNS. Moreover, the studies show that your symptoms are not caused by stress, anxiety or depression.
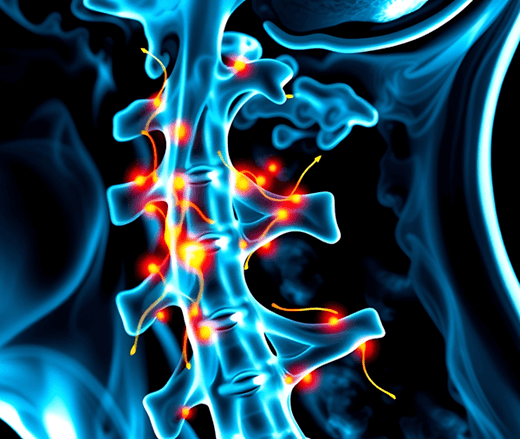
Research focus in fibromyalgia is shifting from the CNS to the periphery. And although your ganglia are at the entrance to your cord (up and down both sides), they are in your periphery and are affected by substances in your blood. If your immune system signals problems, your ganglia can jack up the signals going to your brain. Many researchers believe this is why your brain produces so many fibromyalgia symptoms.
Treatments targeting abnormal brain function are still your best option for taming fibromyalgia symptoms because the immune system’s role needs to be ironed out. It’s unclear if your antibodies, your neutrophils, your gut bacteria, or other components of your immune system are at the epicenter of your symptoms. Then again, a combination of all these factors might be involved.
Getting to the bottom of what is driving your symptoms is essential for you to reap better care. That is why AFSA funded all four investigators mentioned above to advance their research in fibromyalgia patients (not mice).
Stay Current on Treatments & Research News: Sign up for a Free Membership today!
Research holds the key to better treatments. AFSA funded three projects in 2024 and three more in 2025. Help us keep the momentum going.
What is Fibro? | Why So Many Symptoms | Understanding Fibro Flares | CNS Immune Cells
Ganglia & Fibromyalgia Resources
- Hanani M, Spray DC. Nat Rev Neurosci 21(9):485-498, 2020. Free Report
- Gazerani P. Front Pain Res 10:2:646068, 2021. Free Report
- Goebel A, Krock E, Gentry C, et al., 2021. J Clin Invest. 131(13):e144201. Free Report
- Caxaria S, Sikandar S, et al. PNAS 120(17):e2211631120, 2023. Free Report
- Cai W, Minerbi A, et al. Neuron 113(13):2161-2175, 2025. Free Report
Back To Peripheral System Causes
