Treatment & Research News
Tonmya – Novel Fibromyalgia Treatment but Will it Deliver?
Home | Treatment & Research News

All fibromyalgia medications are “dirty” because they soil up your body with unwanted symptoms. Yet, the FDA recently approved a new drug whose mode of action is relatively clean. Tonmya (sublingual cyclobenzaprine) treats fibromyalgia pain, sleep, and fatigue without causing weight gain, dizziness, nausea, dry mouth, sexual dysfunction, foggy brain, and other side effects.
Tonmya’s active ingredient is cyclobenzaprine, a tricyclic drug that has been on the market for 50 years. Oral doses of 10 mg are commonly prescribed for relaxing muscles and aiding with sleep (it’s sedating). But this new drug is not an oral pill that’s swallowed. Tonmya is a sublingual formulation with direct action on the brain to target fibromyalgia sleep and pain. In addition, the dose is only 5.6 mg.
Improving Sleep
With fibromyalgia, you don’t just hurt all over, you have a non-restorative sleep pattern that shows up on an electroencephalogram (EEG). Basically, awake like “alpha” brain waves disrupt your sleep (alpha-EEG) and leave you feeling like roadkill each morning. Fifteen years ago, researchers showed that low doses of oral cyclobenzaprine (3-4 mg) at bedtime reduced the alpha-EEG and mildly improved fibromyalgia pain.1 But despite the low dose, significant side effects occurred (headache, dry mouth, constipation, dizziness).
The above study prompted Tonix Pharmaceuticals to develop and test Tonmya. But they couldn’t just reformulate cyclobenzaprine as a low dose oral pill. A new delivery system was needed to cut the side effects while boosting the drug’s efficacy. Tonix initially tested a 2.8 mg sublingual formula, but it was not potent enough. However, their 5.6 mg sublingual dose shows promise.
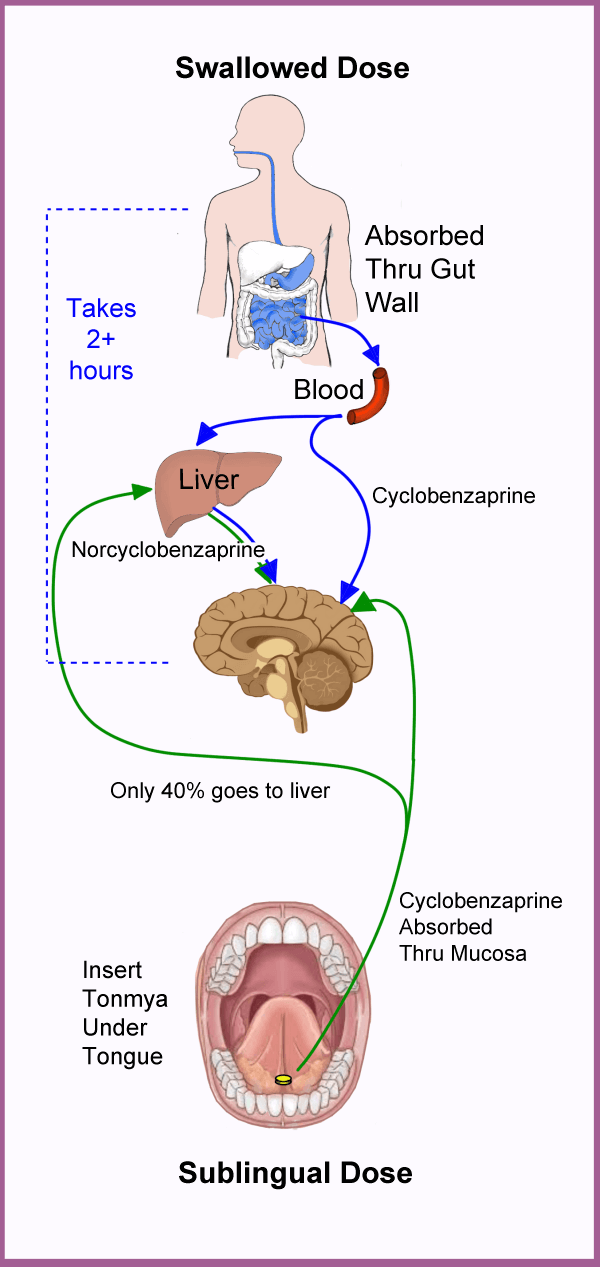
Swallowed Vs Sublingual
Ideally, fibromyalgia patients need a drug that goes straight to the brain to provide symptom relief. That’s the strategy behind Tonmya’s mode of action. It’s quickly absorbed through the mucosa to work fast and maximize the amount of drug reaching the brain.
The swallowed “pill” formula is absorbed through your gut to enter the bloodstream (takes more than two hours). Almost immediately, a large portion is converted to norcyclobenzaprine in the liver. Over time, all the drug is switched to this metabolite and eventually excreted. Unfortunately, norcyclobenzaprine builds up in the blood and causes side effects.
If you take a medication to aid with sleep, blood levels need to peak rapidly and fizzle out by morning. The concentration of swallowed cyclobenzaprine peaks four hours after ingestion and its metabolite remains active for 18 hours. Taking a smaller dose minimizes the metabolite’s daytime side effects (drowsiness, dry mouth, dizziness and fatigue). However, the lower dose may provide diminishing returns on sleep.
Cyclobenzaprine works by blocking the serotonin type 2A receptor. Unlike most serotonin receptors, activating this one leads to more pain while blocking it relieves pain. It also reduces the alpha-EEG sleep disturbance. In addition, the drug inhibits histamine (causing sedation) and acetylcholine (causing dry mouth and nausea). Cyclobenzaprine mildly increases norepinephrine, which contributes to fibromyalgia headaches.
The sublingual formula doesn’t produce gastrointestinal side effects such as nausea or constipation. It also limits the drug’s impact on histamine, acetylcholine and norepinephrine, so the side effects are less. And according to Tonix, the amount of norcyclobenzaprine in the bloodstream is reduced by 40 percent.
Symptom Benefits
The clinical trial data on Tonmya shows it relieved several fibromyalgia symptoms during the 14-week study.2 Based on 457 patients (half taking a placebo), the sublingual reduces pain, sleep disruption, fatigue, cognitive impairments (fibrofog), and sexual dysfunction. But not everyone responded to Tonmya, so what are the odds it will help you? And how long do you have to take the drug to determine if it works? The answer to the latter question is two weeks.
About one out of five fibromyalgia patients will notice a 30 percent drop in pain while taking Tonmya. The odds drop to one out of ten for a 50 percent reduction in pain. Your improvement odds for the other symptoms are hazy due to the way the data is presented. However, the differences between the drug and placebo group are highly significant for all symptoms. Sadly, not enough men were enrolled in the study to know how they respond to Tonmya.
Side Effects
The common side effects of swallowed cyclobenzaprine are plenty (see above), but what about Tonmya? Most problems occurred in the mouth such as numbness (24%), product abnormal taste (12%), tingling (7%), and tongue discomfort (7%). The systemic effects of headaches and daytime sleepiness only occurred in 2%.
Given the high incidence of oral cavity symptoms, did this unblind the participants? In other words, did the oral cavity side effects lead fibromyalgia patients to suspect they were taking Tonmya and report greater symptom benefits? No. The drug improvements were the same for those who noticed oral symptoms and those who didn’t.
Unlike pregabalin, Tonmya doesn’t cause weight gain or daytime fatigue. Nor does the drug cause sleep disruption, cardiovascular symptoms (blood pressure elevations), or gastrointestinal issues (nausea and constipation). The latter are common side effects associated with duloxetine and Savella. Then again, more Tonmya side effects could crop up after thousands of fibromyalgia patients try it.
Availability and Cost
On November 17, 2025, doctors in the United States began writing prescriptions for Tonmya. For the time being, a 30-day script consists of 60 sublinguals containing 2.8 mg of the drug (you will need to take two per night). Hopefully, Tonix will manufacture the 5.6 mg dose so that patients will only require one sublingual. The point is, don’t be fooled by the quantity on your prescription (and watch out if you only get 30).
Unless your insurance company covers the cost of Tonmya, it will set you back $1,900 per month. And even if you do have coverage, Tonmya will likely be placed into the most expensive price tier. Get a price quote before you end up in a pharmacy checkout line (remember, you need two 2.8 mg sublinguals).
When pregabalin, duloxetine, and Savella were first FDA-approved, most insurance companies baulked at coverage. Many outright denied paying for the drugs. Others put these medications into the most expensive tiers (brackets) in their formularies. But if Tonmya is truly as good as it sounds for treating fibromyalgia, the insurance industry will soften up.
Looking Ahead
The clinical trial for Tonmya involved less than 500 fibromyalgia patients. What happens when the drug hits the commercial prescription market and is put to the test by thousands of patients? Will the drug live up to its promise to relieve your symptoms without serious side effects? Let’s hope so because you deserve effective treatments.
Join AFSA and stay connected for updates on the rollout, including cost/insurance issues and patient response to Tonmya. We will interview fibromyalgia treatment experts and get their feedback. Chances are the FDA guidelines for taking the drug (dosing and timing) won’t be optimal for you. And sometimes, new side effects emerge after thousands of patients take a medication.
Don’t Miss a Beat on New Treatments & Research: Sign up for a Free Membership today.
Research holds the key to a better life. AFSA funded six projects in the past two years; help us fund more!
What is Fibro | Treating Fatigue & Brain Fog | Diet & Nutrition | Mondrug Treatments | Muscle Pain Relief | Lifestyle Changes
Tonmya Treats Fibromyalgia References
- Moldofsky H, et al. J Rheumatol 38(12):2653-63, 2011. Free Report
- Lederman S, et al. Pain Med doi: 10.1093/pm/pnaf089. Online ahead of print. July 8, 2025. Free Report
