Why Fibromyalgia Muscles Hurt
Treatment & Research News
Why Fibromyalgia Muscles Hurt
Home | Treatment & Research News

Hurting all over and feeling stiff as a board, it’s hard to fathom your muscles are not the source of your discomfort. Yet, the popular theory on your disease says the pain processing centers in your brain are the cause of your grief, not your muscles. But new research by Robert Katz, M.D., and Frank Leavitt, Ph.D. of Rush University in Chicago, explains why the muscles are an important source of fibromyalgia pain and stiffness.1,2
Katz and Leavitt found the pressure inside the muscles of fibromyalgia patients is three times higher than normal. In fact, the greater the pressure, the higher the patient’s pain ratings. What’s more, this discovery explains many of your symptoms and offers a different viewpoint on treatment.
Beneath the Surface
Pressing on your muscles elicits pain but says nothing about why they hurt. Biopsies of the muscles beneath the surface show subtle abnormalities yet fail to explain your pain. “Researchers are using the wrong measuring stick,” says Leavitt.
A handheld gauge with a needle tip can be inserted into the top shoulder muscle (trapezius). It works like a barometer to measure the fluid pressure inside the muscles and this value is increased by a factor of three in people with fibromyalgia.
If you feel like your muscles are squeezed so tightly that they hurt – like the cuff on a blood pressure kit – this finding confirms your suspicions. In addition, the excessive pressure compresses your blood vessels, impairing the delivery of nutrients and oxygen. Impaired oxygenation (hypoxia) messes with muscle function. Other studies document hypoxia in fibromyalgia patients but with no clear explanation.3,4
“Oxygen deprived muscle tissue brought on by internal pressure is an important source of muscle pain in fibromyalgia,” says Leavitt. He adds, it’s a paradigm shift from assuming all pain is generated by malfunctions in the central nervous system. “This dramatic change in our understanding opens the door to fresh perspectives on treatment.”
“In our view, myalgias are associated with elevated muscle pressure rather than tissue damage or central nervous system factors,” says Katz and Leavitt. Although current treatments focus on the nervous system, the researchers suggest targeting the muscles in addition to the brain.
Wondering what causes elevated muscle pressure? High levels reflect increased muscular activity (or tension). In other words, your muscles are chronically activated even when you are not moving.5
Sex & Age
Pressing on the muscles is used to assess tenderness, but men have higher thresholds for this test. In fact, this was the drawback of the old diagnostic criteria for fibromyalgia because it failed to identify many men with the disease. But the situation is different when measuring the pressure inside the muscles.
The average pressure was the same for both the men and women in the study. Separating the patients into three age groups also did not reveal any differences.
A Fibro Marker?
Only 2 of 142 fibromyalgia patients had pressures in the control group range. There was almost no overlap, despite the controls having chronic pains (such as rheumatoid arthritis and lupus) which are often confused with fibromyalgia.
Referring to the threefold difference in pressure between the two groups, Leavitt says, “We are not dealing with small innocuous amounts. This is obvious medical evidence of abnormalities in the peripheral system, and it has gone unnoticed for decades. In addition, changes in pressure were accurately predictive of changes in the perception of pain, with increased pressure consistently associated with increased pain.”
Measuring muscle pressure has two downsides: it’s invasive and not generally available in doctor’s offices. However, it may prove to be a useful research tool.
Symptom Validation

A substantial increase in muscle pressure explains why you have so many fibromyalgia symptoms, especially those related to muscle pain. It lends support for feeling like your body is in a pressure cooker–about to blow a gasket—or that your muscles are bursting at the seams. Aside from pain, high muscular pressure could be the source of the following symptoms:
- Inability to relax your muscles and get comfortable
- Your extremities feel swollen (even though they aren’t); it is the sensation that your muscles have solidified like Jello
- Body-wide stiffness unrelated to your joints
- Trouble initiating an exercise program (because your muscles are tight and deprived of oxygen); regular movement helps but if you stop for a few days, you are back at square one
- Cold extremities, muscle fatigue and cramps (due to blood flow restrictions and hypoxia)
Targeting Your Muscles
Massage, acupuncture, heat, and various physical therapy techniques reduce muscle tension and improve circulation. Quite possibly, these approaches work (at least in part) by reducing the pressure build up inside your muscles. Even the advice to keep moving throughout the day and perform gentle exercise may reduce the pressure in your tissues.
Close to 15 percent of the fibromyalgia patients enrolled in the above study were retested at a follow-up visit. Those reporting a reduction in pain exhibited a corresponding drop in muscle pressure. “One of my research goals,” says Katz, “is to determine if muscle pressure can be reduced by various treatments.”
![]()
“I’m hoping to validate the use of muscle relaxants to decrease muscle pressure in fibromyalgia patients,” says Katz. “There are several possibilities, but I rely on cyclobenzaprine and tizanidine. In addition, it’s important for patients to sleep through the night and rest their bodies, so I prescribe amitriptyline, doxepin, or trazodone.”
Leavitt points to another treatment avenue: dilate the blood vessels to increase the supply of oxygen to the muscles. Examples include magnesium and Co-Enzyme Q10. Perhaps it is no surprise that a 2024 study found low magnesium levels are linked to greater muscle stiffness in people with fibromyalgia.6
“If muscle pressure is the reason why fibromyalgia patients have so much pain, we should be open to different treatment strategies,” says Katz. “Despite the fact that 12 million people in this country have this disease, and often undiagnosed, there is plenty of room for experimentation.”
Credibility
“For many clinicians,” says Leavitt, “pain behaves as an indicator of underlying abnormalities at the site of pain. In fibromyalgia, this expectation is not met. But elevated muscle pressure supports the presence of an identifiable pathology. Patients may no longer have to face the credibility conundrum of intense pain and essentially normal laboratory findings.”
Treatment Help
Several approaches are briefly described below with links for more information.
Muscle Relaxants – This group of medications fall into a variety of classes. Three fibromyalgia experts offer advice on selecting a relaxant, dosing up, and overcoming side effects.
Massage – This nondrug therapy is top-rated by fibromyalgia patients to ease muscle pain.
Heat Wraps – Hot tubs, baths or showers are great for relieving discomfort, but you can’t immerse your body in water all day long. Learn how heat wraps treat fibromyalgia pain.
Physical Therapy Techniques – Therapists use a variety of approaches to ease your muscle tension and improve circulation.
Fibro-Friendly Exercises – In the short term, implementing a new exercise program will make your muscles ache more. But in the long run, regular movement therapy reduces pain and gives you more strength to make it through the day. Learn a few easy steps to make your muscles more resilient.
Lifestyle Changes – Read about self-help strategies can you use to minimize the strain on your sore muscles.
Magnesium – The typical oral dose is 500 mg/day. Or you may prefer an oil-based magnesium that you can spray on sore muscles to help them relax.
Co-Enzyme-Q10 – Try a dose of 100 mg/day. This enzyme must be purchased as oil-based gel caps and it’s very expensive, so shop around.
If you are perplexed as to why you have so many symptoms in addition to muscle pain, see What Causes Fibromyalgia. Your central nervous system (brain and spinal cord) does a poor job of controlling pain and alterations in other body systems make matters worse. What can you do? There are medications that boost your pain inhibitory controls as well as those that aid with sleep.
Talk to your doctor about treatment options; they can help with referrals to physical therapists and acupuncturists.
Stay Current on Treatments & Research News: Sign up for a Free Membership today!
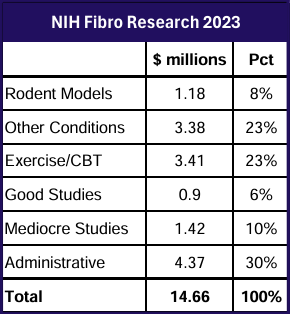
Research holds the key to better treatments. AFSA funded three projects in 2024; help us fund more in 2025.
Diagnosis | Symptoms | What Is Fibro | Finding A Doctor | Muscle Pain Relief
References for Why Fibromyalgia Muscles Hurt
- Katz RS, Leavitt F, et al. J Rheumatol 48(4):598-602, 2021. Free Report
- Katz RS, et al. J Clin Rheum 30(2):79-83, 2024. Free Report
- Villafaina S, Parraca JA, at al. Biomedicines 11:132, 2023. Free Report
- Shang Y, et al. Arthritis Res Ther 14(6):R236, 2012. Free Report
- Ates F, et al. Front Physiol 10:196, 2019. Free Report
- Tarsitano MG, et al. Eur Rev Med Pharmacol Sci 28(14):4038-4045, 2024. Free Report